Can Alien Life Be Silicon Based?
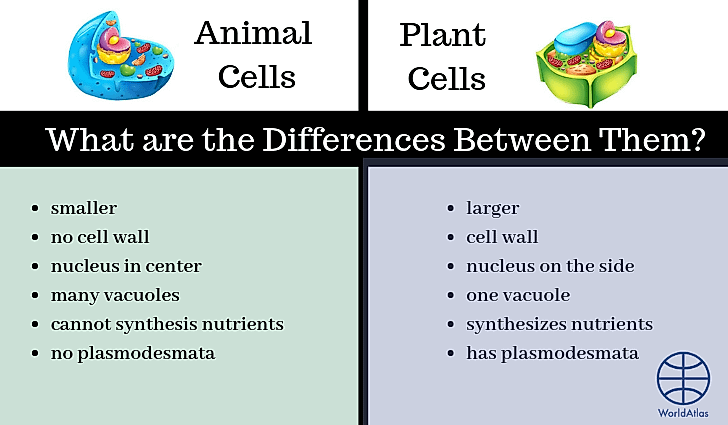
- All life on Earth is carbon-based
- Silicon has many similarities to carbon, suggesting it could be used in the formation of life
- Silicon is different enough from carbon that it may not be possible for silicon-based life to exist
All forms of life on Earth are carbon-based. Carbon forms the backbone of all organic chemistry, and that includes the DNA molecule. Without carbon, life on Earth would not be possible. Or at least, life as we know it. Although every organism is carbon-based on Earth, would it be possible for forms of life to arise and evolve that are based on a different element? If life can form from other means, the most likely element would be silicon. Silicon is located just below carbon on the periodic table, meaning that like carbon, it can form up to four individual bonds at once. If any element other than carbon could give rise to life, it would be silicon. Is it possible for silicon-based life to exist somewhere in the universe?
Why Is Life On Earth Carbon-Based?

Carbon is one of the most common and unique elements in the universe. Carbon is able to form up to four bonds at once, which allows it to form hundreds of different molecules. The type of molecules that carbon can make are important for life on Earth. For example, plants take in carbon dioxide and produce oxygen. When we and other organisms eat food, we produce carbon-based molecules that give us energy. When we begin to understand how carbon interacts with living organisms, it is not surprising that it forms the basis for all life on Earth. In order to determine whether or not silicon could replace carbon, it is important to determine whether or not silicon can do everything that carbon can. Interestingly, silicon is actually far more abundant on Earth than carbon, which suggests that if life can be silicon-based, the conditions on Earth may not have allowed for it. Or perhaps silicon-based life did form alongside carbon-based life, yet carbon-based life simply outcompeted it for resources.
Can Silicon Replace Carbon?
Silicon and carbon have many similarities, yet they also have many differences. Both elements are capable of making up to four bonds at once, yet the strength of those bonds is different. For carbon, each of its four bonds are similar in strength, which makes it easy for living organisms to perform chemical reactions and swap different molecules. For silicon, however, the four bonds vary wildly in strength. The first bond silicon forms is far stronger than any subsequent bonds, making it far more difficult to perform chemical reactions with silicon. Furthermore, silicon cannot produce the same molecules as carbon, and so many of the life-supporting chemicals we rely on would not be possible with silicon-based life. For example, plants take in carbon dioxide and release oxygen, with the carbon being used to create food for the plant. If plants were silicon based, they would not be able to absorb carbon dioxide and they would have no way of producing energy for themselves.
Interestingly, one of the reasons why silicon-based life never formed on Earth despite its abundance is that silicon is a fairly inert element under the Earth’s surface temperature. In order for silicon to be used in various chemical reactions, the temperature has to be far higher. Thus, silicon-based life would have to form under far hotter conditions. However, under such high temperatures, there would be no liquid water, another essential ingredient for life. Even if another compound, such as ammonia or methane, could be a solvent in liquid form, temperatures would still be far too high for them to exist in liquid form.
Silicon does not seem nearly as promising as carbon when it comes to forming life, yet experiments have shown that carbon-based life can incorporate silicon instead of carbon for some chemical reactions. However, although silicon can be incorporated into some organisms, this would be more of a silicon/carbon-based form of life rather than just silicon-based.
Is Silicon-Based Life Impossible?
Given all this information, it seems as though silicon-based life would be impossible. Things may not actually be so simple. Silicon-based life may be impossible on Earth, yet we really don’t know if it would be possible under different conditions. Earth-life is the only example of life we have, and so our understanding of life is clearly biased. Alien life could be so alien to us that it could be silicon-based and use entirely different means of survival. In the search for life beyond Earth, it might be a good idea to expand our search beyond our biases.