Do Water And Ice Weigh The Same?
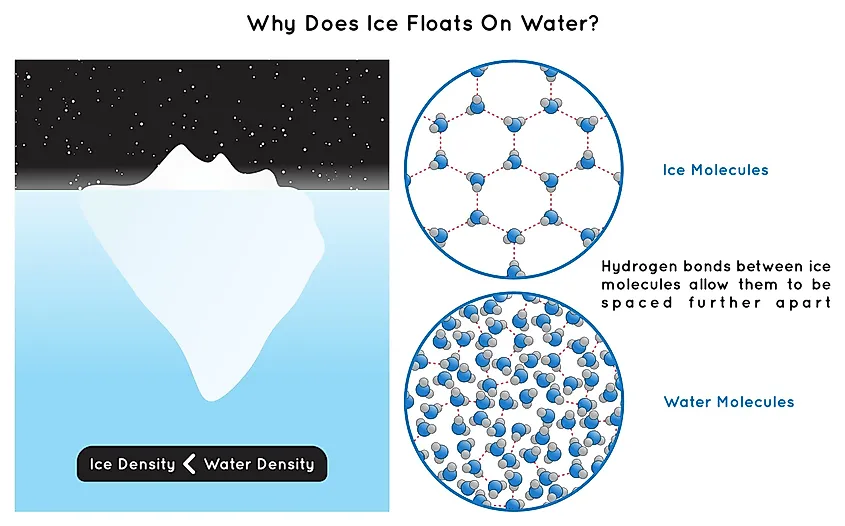
When water freezes into ice, its molecular structure undergoes a transformation; molecules rearrange into a crystalline structure, causing a roughly 9% increase in volume. This expansion is a result of the transition from liquid to solid, and it reduces the density of ice compared to liquid water.
Despite their different physical properties, both liquid water and ice maintain the same chemical composition. Consequently, the total mass remains constant when water freezes: the mass of the water before freezing equals the mass of the ice afterward. This conservation of mass is a fundamental principle of physics; however, conservation of volume is not. Thus, due to the increase in volume, 1 liter of ice is less dense and thus weighs about 9% less than 1 liter of water.
The Density Of Water And Ice
Consider you have a bucket that contains one pound of water. If you were to freeze all the water in that bucket, would the weight of the water change or stay the same? According to the law of conservation of mass, after the water freezes, there will still be one pound of water since no water has been created or destroyed. All that has occurred is a change from a liquid state to a solid one. Thus, the weight will certainly not change.
However, ice is less dense than liquid water, a property that is unique to water. Having a lower density means that ice floats when placed in liquid water. When water freezes, it occupies more space than in its liquid form because its molecules expand. Therefore, if we have 1 liter of ice and 1 liter of water, the water will weigh more because it is denser.
How The Density Of Water Affects Life

Water's unique property of being less dense as a solid profoundly impacts life on Earth. For example, in winter, when water freezes, it forms a layer of ice on top of liquid water, as seen in Lake Baikal, Russia, where frozen layers form due to cold winter temperatures. This floating ice layer insulates the liquid water below, helping maintain relatively stable temperatures that are crucial for aquatic life to survive the winter months.
If ice were denser than liquid water, it would sink as it forms, leading bodies of water like lakes, rivers, and even oceans to potentially freeze from the bottom up during cold spells. Such a scenario would disrupt aquatic ecosystems and could lead to the extinction of many species, as ice formation would eliminate the thermal refuge necessary for survival. Fortunately, because ice floats, water bodies can support life throughout harsh conditions. This remarkable property of water, often taken for granted, plays a critical role in sustaining life on Earth. Every time you observe ice floating on a lake or in a glass, remember that this simple phenomenon is a fundamental reason life thrives on our planet.