What is Maxwell's Demon?
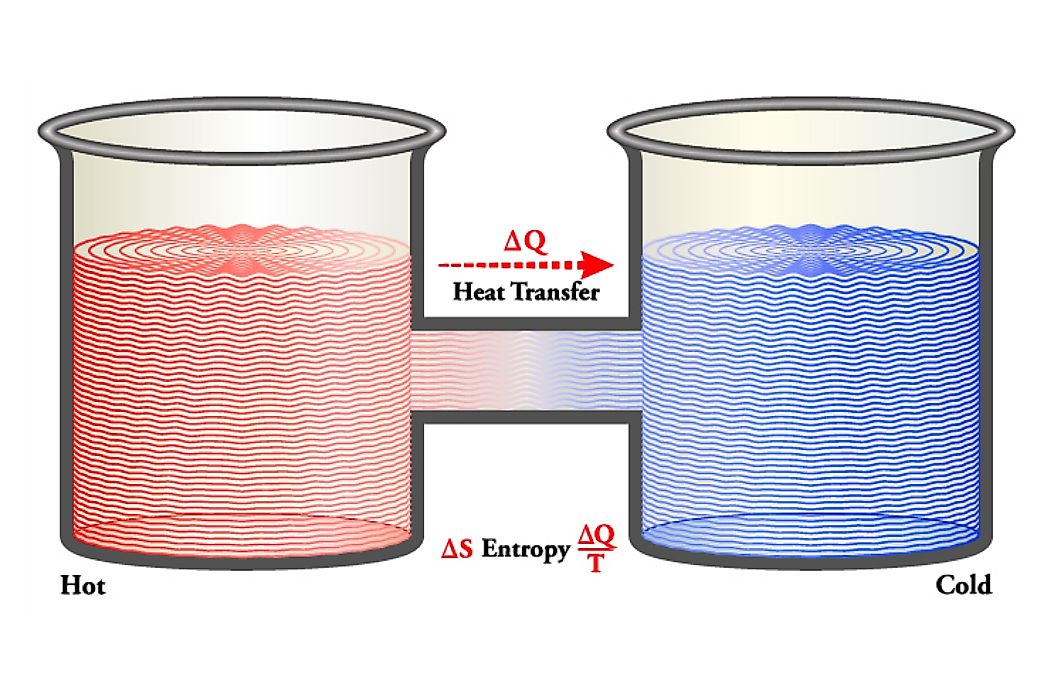
The concept of Maxwell’s Demon is named after a James Clerk Maxwell. The concept seeks to explain what happens when two objects are subjected to different degrees of heat. The concept that a demon opens door to fast-moving particles making them move to one chamber consequently making it hotter than the other chamber is still being widely researched. The idea has violated the second law of thermodynamics.
Origin and History of Maxwell's Demon
The idea was first floated in December 11, 1867, by Maxwell in a letter to Peter Guthrie Tait, his fellow scientist. In 1871, Maxwell again wrote to John William Strutt on the same idea. With their input, Maxwell presented the idea to the public in 1972 through a book titled “Theory of Heat.” The name “demon” was first used by William Thomson in the “nature” newspaper in 1874. His research was based on the two laws of thermodynamics. The first law states that energy can be neither be “created nor destroyed” while the second law states that once energy is converted it is irreversible.
Maxwell’s Experiment
Maxwell used two vessels; vessel X and Y with gas inside. The vessels were placed next to each other and a small hole drilled between them. Temperatures were then either lowered or increased in either of the vessels. The molecules in one vessel tended to move depending on the adjustment initiated as a result of acquiring or losing energy. He imagined that a demon guarded the doorway and separated the molecules moving at a high speed from those moving at average speed.
When a fast-moving molecule from X shoots in the direction of the trapdoor, the door is opened by the demon, and the molecule will move from X to Y. The same way when a slower moving molecule from Y rushes towards the trapdoor, the demon will let it pass from Y to X. The approximate speed of the molecules in Y will have increased while in X they will have gradually reduced. Since approximate molecular speed corresponds to temperature, the temperature decreases in X and increases in Y, contrary to the second law of thermodynamics. The demon must allow molecules to pass to either side so as to produce only a temperature variation. The experiment found that the gate separating the two sections opened selectively depending on the speed of the particles.
Criticism to the Experiment
In 1929, Leo Szilard and Leon Brillouin wondered how the “demon” was able to measure the speed of the molecules. They argued that the theory could not eliminate the thermodynamic rule. They also asserted that the “demon” will, with time, lack space to store the information hence begin erasing the previously stored data. Scientifically it is not possible to get out more energy than you put in. The process of opening and closing the “demon” gate requires some energy consumption. This means that to identify the hot or cold molecules, more energy is required making Maxwell’s idea questionable since it would trigger the opening of the gate even for cold molecules.