What Is Spectroscopy?
The science of spectroscopy is one of the most important areas of astronomy. Spectroscopy is the study of an object’s composition by examining the light it emits. Light emitted by an object such as a star contains the information of what that star is made of. To understand how light contains such information, we must understand the behaviour of electrons and atoms.
Electron Energy Levels
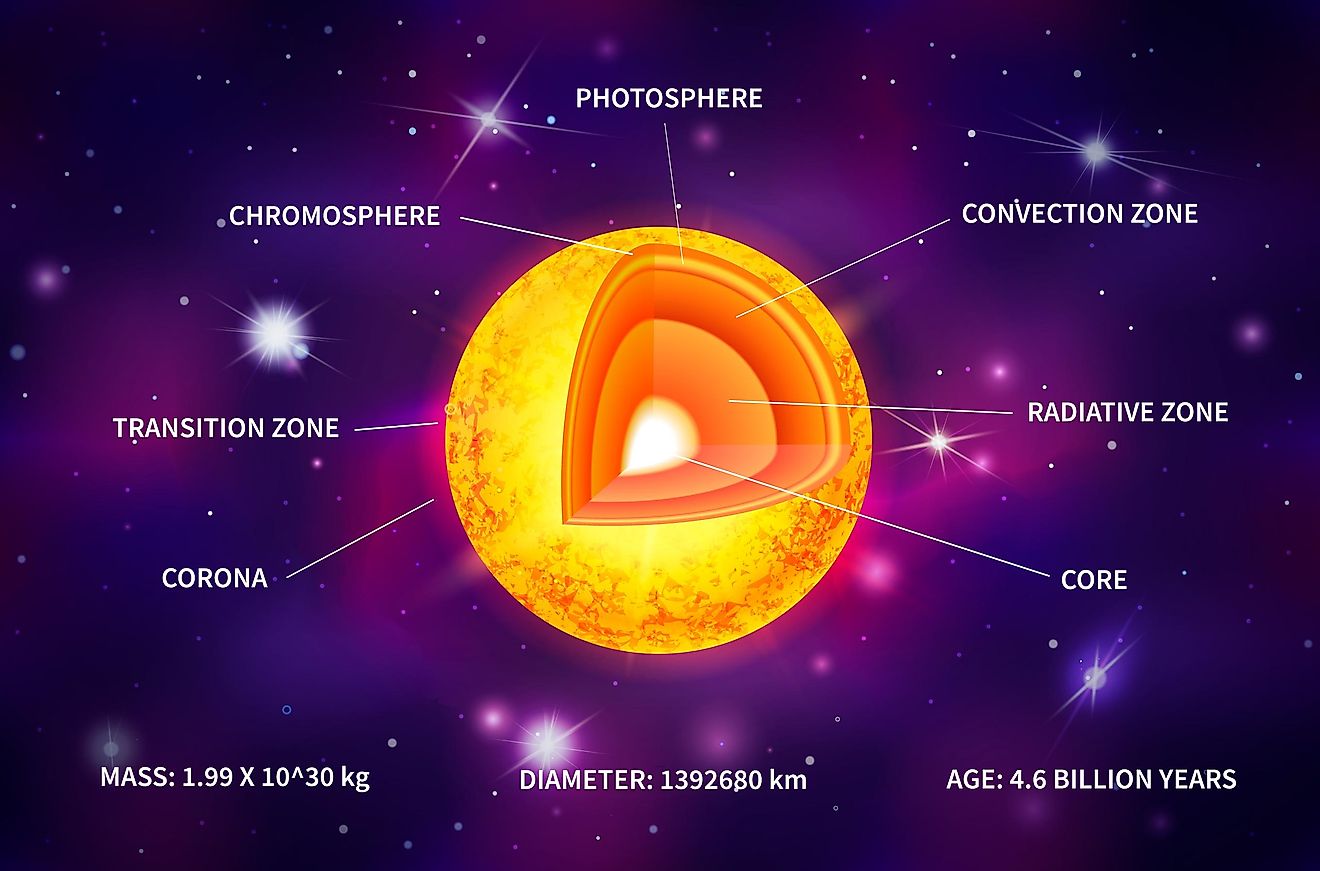
Most of us are likely familiar with the basic model of an atom. You have a central nucleus containing protons and neutrons, with electrons orbiting the nucleus. A more accurate model, however, is to think of electrons not as objects orbiting the nucleus in a way similar to planets around the sun, but rather, to think of it as an electron cloud. Electrons do not orbit the nucleus along a singular path. Rather, electrons exist at varying energy levels. Electrons can jump from one energy level to another, either moving to a higher energy level or falling to a lower energy level. In order for an electron to move to a higher energy level, it must be hit by a particle of light called a photon. When a photon hits an electron, that electron will absorb the photon and its energy, allowing the electron to jump to a higher energy level. To fall to a lower energy level, that electron will emit that same photon back into space.
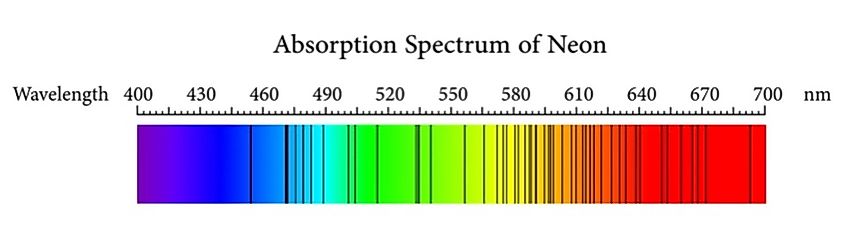
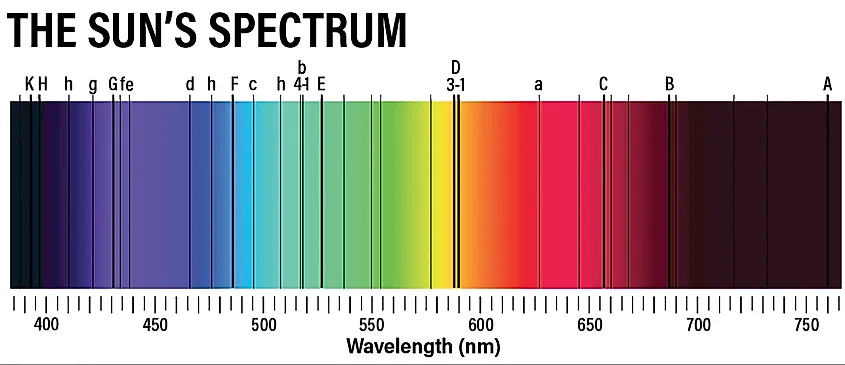
Interestingly, different atoms will have different electron energy levels. For example, hydrogen has different energy levels than oxygen. The different energy levels between atoms means that the photons emitted by their electrons will all have different amounts of energy. Thus, depending on the energy of the photon, we can determine what atoms emitted those photons. Using what’s called a spectroscope, scientists can split a beam of light into its constituent colors and analyze those colors for any dark bands. The dark bands are where electrons absorb photons, allowing scientists to determine what types of atoms are absorbing those photons.
What Objects Can We See The Composition Of?
Stars are the primary sources of light in the universe, and so spectroscopy generally involves starlight in one way or another. When a star emits light, that light has to pass through the star’s atmosphere. Atoms in the atmosphere will absorb and emit the star’s photons, allowing astronomers to determine the composition of the star. Furthermore, certain types of nebulae emit their own light, allowing scientists to determine their composition as well. Perhaps one of the more interesting applications of spectroscopy is the analysis of planetary atmospheres. As planets pass in front of their star, light from that star has to pass through the planet’s atmosphere. The star’s photons will be absorbed by the atoms in the planet’s atmosphere and then emitted back into space. Here on Earth, we can capture those photons in a spectroscope and determine the composition of the planet’s atmosphere. As this technology develops, we may soon have the ability to look for signs of life in the atmospheres of other planets.