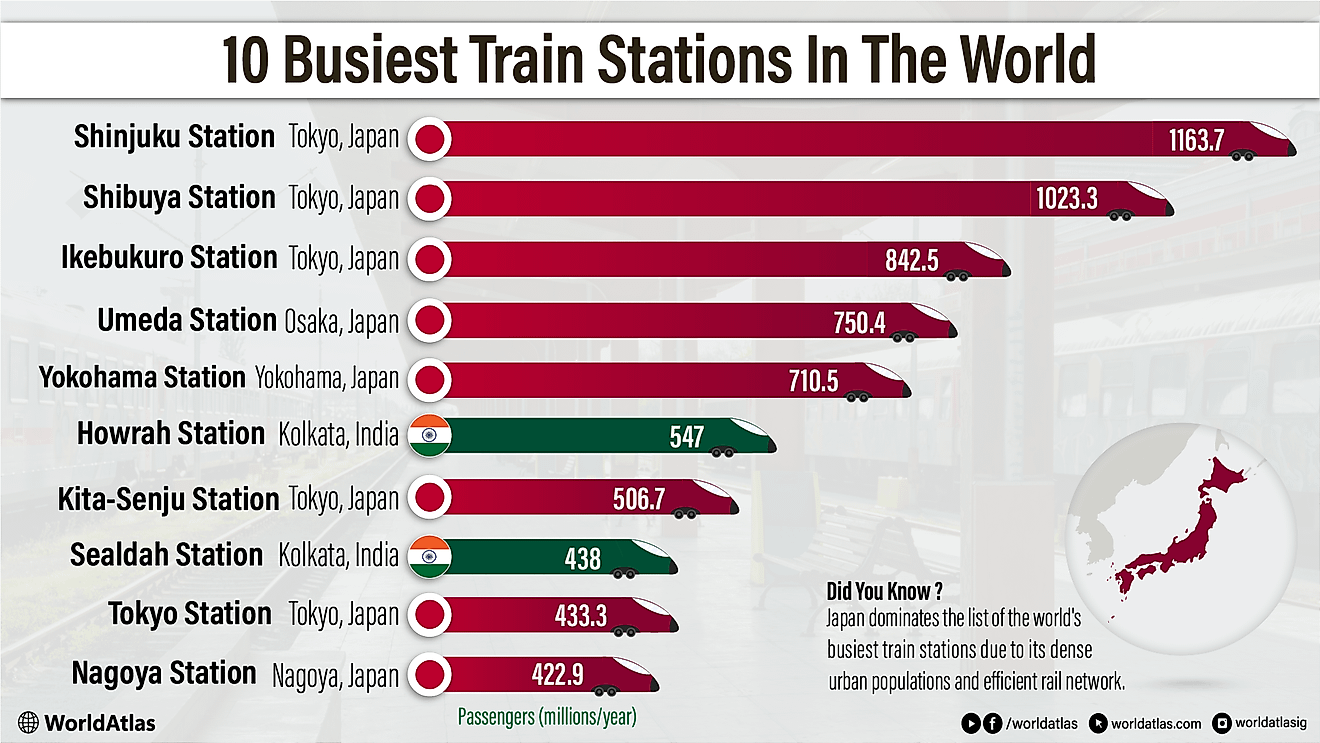
Muriatic Acid
Hydrochloric acid is known by other names including acidum salis, spirit of salts, and muriatic acid. The term “muriatic” means “pertaining to salt or brine.” Muriatic acid is a strong corrosive acid known for its distinctive pungent smell. It is chlorine-based acid that contains water. The acid is a solution of water, hydrogen chloride, and other chemical species such as chloride and hydronium ions. Muriatic acid is an important industrial chemical used in the production of plastics. It is also used at home as a descaling agent as well as a food additive in the food industry.
Discovery And Development
Hydrochloric acid was possibly discovered around 800 AD by alchemist Jabir ibn Hayyan. In the 13th century, a widely known manuscript about alchemy called the Pseudo-Geber described preparing aqua regia by dissolving hydrochloric acid in nitric acid. Free hydrochloric acid and its preparation were first described in detail by Andreas Libavius in the 16th century when he prepared the acid by heating salt in a container made of clay. Other sources claim that the pure acid was discovered in the 15th century by Basil Valentine when he heated green vitriol and common salt. By the early 19th century, it was proven that the chemical composition of the acid contained chlorine and hydrogen.
Industrial Revolution
During the Industrial Revolution, soda ash production was quite widespread, especially in Europe. The Leblanc process was a method of producing soda ash that involved the use of sulfuric acid, coal, and limestone, resulting in hydrogen chloride as a byproduct. The byproduct was vented into the air, leading to pollution. In 1863, Britain passed the Alkaline Act and similar legislation was also passed in other countries. The legislation required producers of soda ash to absorb excess hydrogen chloride in water. The move led to the production of muriatic acid on an industrial scale.
Chemical And Physical Properties

Hydrochloric acid is prepared by treating hydrogen chloride (HCL) with water (H2O) resulting in this chemical equation:
HCL + H2O H3O+ + CL-
Muriatic acid is a strong acid because of its complete dissociation with water. It is one of the six strong mineral acids and is usually referred to as monoprotic acid because it is least likely affected by the oxidation-reduction process. Hydrochloric acid is less hazardous compared to other strong acids since it contains non-toxic and non-reactive chloride ions. It is the preferred acid in titration to determine the concentration of bases. Its physical properties such as density, boiling point, melting point, and pH depend on the concentration of hydrogen chloride in water. As a binary mixture of water and hydrogen chloride, the acid has a constant boiling point mixture at 20.2% hydrogen chloride and 108.6 °C.
Uses Of Muriatic Acid
The use of muriatic acid in the industrial process is often determined by the required quality of the product. One of the most common uses of this acid is in the pickling of steel to get rid of rust before further processing. Another important use of the acid is in the production of organic compounds for polyvinyl chloride plastic. Hydrochloric acid is also used to control or neutralize the pH of a solution. Other uses include household cleaning, leather processing, and the production of narcotics such as cocaine and heroin.