What Is an Isotope?
What Is an Isotope?
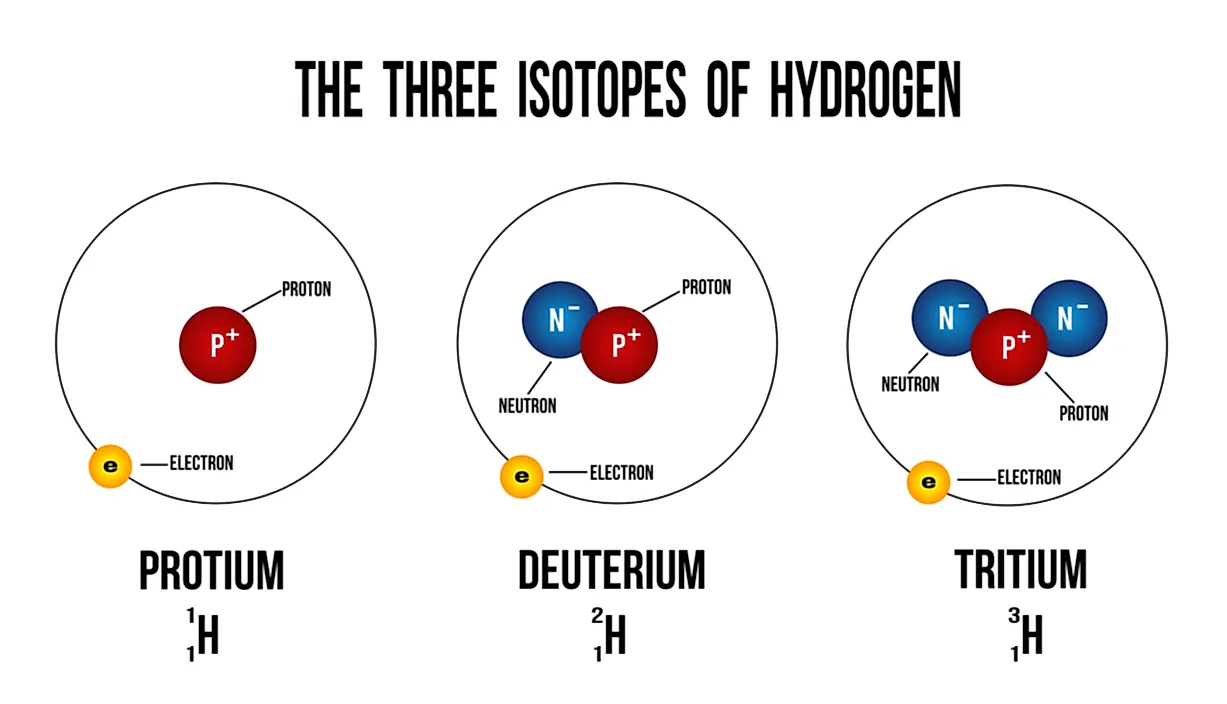
Isotopes are atoms of the same element that have an equal number of protons but different numbers of neutrons. Their atomic numbers are the same, but their mass numbers are different. Mass numbers are always denoted by A, whereas Z refers to the atomic numbers of elements. The atomic number symbolizes the number of protons in an atom's nucleus, and is used to identify the position of the element on the periodic table. The mass number of an atom is the number of neutrons in its nucleus. The isotopes of elements have different physical properties due to the variation in their atomic masses. Due to this difference, such isotopes have different densities, as well as melting and boiling points. However, isotopes of an element always have very similar chemical properties. The similarity occurs because only the electrons are used in chemical reactions, not the neutrons or protons.
History of Isotopes
Radiochemist Fredrick Soddy first suggested the existence of isotopes in 1913 after conducting studies that involved the decay of radioactive chains. During his experiments, Soddy realized forty different species existed between lead and uranium, yet the periodic table could only accommodate 11 atoms. After chemical tests conducted to separate some of these elements had failed, he suggested that more than one atom types could share the same position in the periodic table and named them isotopes.
Examples of Isotopes
Chlorine contains two major isotopes: chlorine-35 and chlorine-37. To arrive at this conclusion, scientists have discovered that in a chlorine substance, proportions of each of these isotopes exist overall, and that is why ratios are used to express the difference in quantity. These ratios are helpful when calculating relative percentages and relative atomic masses. Other examples of isotopes include carbon (carbon-12 and carbon-14 isotopes), oxygen (oxygen-16 and oxygen-18), and phosphorus (phosphorus-31 is the primary isotope, though specific amounts of phosphorus-32 also exist). The isotopes of these compounds are considered stable, and most of them have only two isotopes. However, there are a few elements that have just one isotope, and these include fluorine, beryllium, arsenic, yttrium, gold, aluminum, iodine, manganese, sodium, and niobium.
Purification of Isotopes
There are three main areas where isotopes are applied. The first is the separation of isotopes. Separation facilitates maximization of the properties of the atoms as required. In the separation of lighter elements such as deuterium and oxygen, there is the application of the gas diffusion method. Separation of heavy elements such as uranium and plutonium occurs by mass spectrometry.
Application of Isotopes
The first application of isotopes is its use by archaeologists in carbon dating. Isotopes are two kinds: stable and radioactive isotopes. Stable isotopes contain equal combination of protons and neutrons and as such do not undergo decay. On the other hand, the radioactive isotopes have unstable nuclei and thus undergo decay. The radioactive decay may take as long as 5,730 years, such as the carbon element. Archaeologists use this component of isotopes to determine the age of an object found in archeological digs.