Ocean Acidification

- Ocean acidification refers to the lowering pH levels of the Earth's seas.
- Carbon emissions are the leading cause of acidification.
- Acidic oceans can be very harmful to marine life, leading to ecosystem collapse and wider negative effects on human life.
Ocean acidification refers to the process by which the Earth’s oceans have become more acidic over time. A measurement known as pH quantifies how acidic or basic water is, with a range from 0 - 14.
What Is Ocean Acidification?
Ocean acidification is the lowering of the pH level in the ocean and sea water collectively around the world. This change in chemical properties and acidity levels can be detrimental to marine plants and animals that rely on specific conditions to thrive.
The increased acidity in the ocean is mainly due to higher levels of carbon dioxide. Roughly 30% of the excess carbon dioxide released into our atmosphere is absorbed by the Earth's seas. While this may not sound significant, even a slight change in the balance of pH and chemicals (natural or unnatural) within the Earth's seas can throw off an ecosystem, or even lead to that system’s collapse. Without balanced, fully functioning marine ecosystems, biomes and biospheres are in danger and there can be resonating effects on all other parts of the Earth, including direct effects on human life.
How Did It Begin?

Human industrialization saw its first boom some 200 years ago. Since then, use of fossil fuels and the production of excess carbon dioxide has been an ongoing issue. As human development has increased, so has the level of pollutants and gases which are released into our atmosphere. Similarly, deforestation and agriculture have caused a significant shift in the types of gases released, as well as removed large areas of vegetation for our once lush planet.
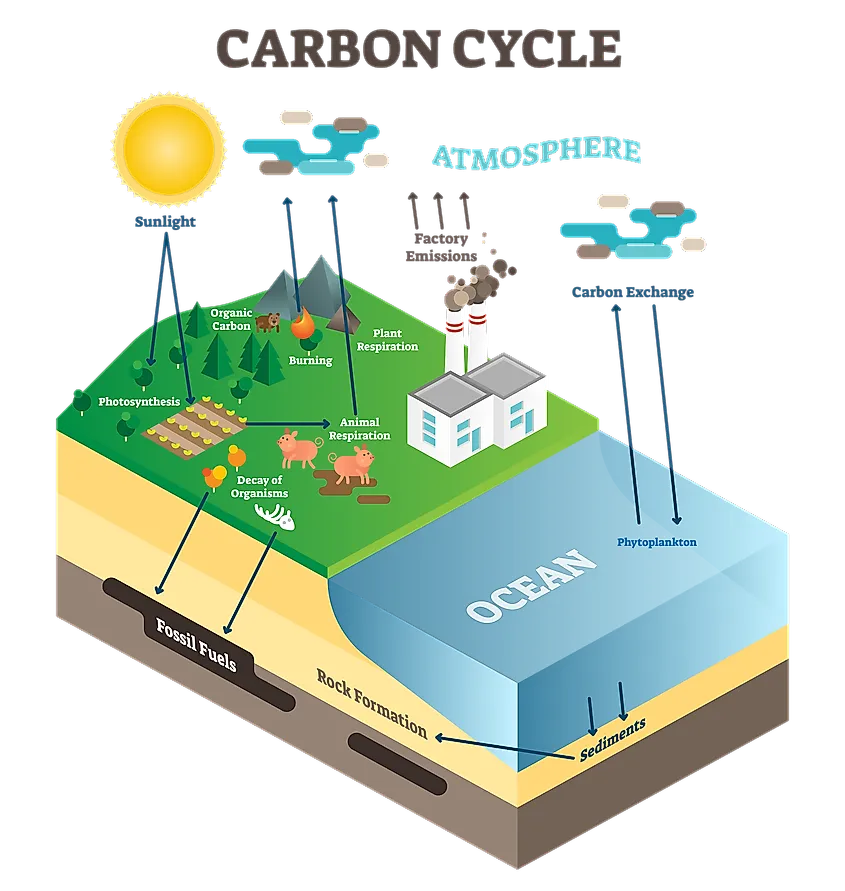
The biggest concern when it comes to burning fossil fuels is the increased output of CO2. Forests, which are natural carbon sinks, absorb this CO2 and help keep the Earth in balance. However, as carbon dioxide biproducts have increased, forests have been cleared and cut, meaning there are less and less trees to help filter and retain this excess carbon. As a result, carbon is released into the air, and rather than being absorbed by vegetation, it is absorbed by the oceans. The oceans can absorb a large amount of carbon dioxide, however it is not without significant side effects. Chemical reactions caused by the addition of carbon alter the ionic balance of the water. This results in a more acidic solution, thus the ‘acidification’ of the Earth’s seas. The results of this increased acidity are vast and dangerous.
The Chemical Process: An In-depth Look
Acidification is the simple term for a much more complicated chemical process. First, carbon dioxide, which is carbon and oxygen, dissolves in water which is composed of hydrogen and oxygen. This results in a carbonic acid known as H2CO3 which is a compound of all three chemicals. This is an acidic solution which breaks apart into both hydrogen ions and bicarbonate ions, HCO3-.
What Causes Acidification?

Because acidification is caused by an increase in carbon dioxide, any activity that results in carbon emissions can affect ocean acidification. One of the main causes is the burning of fossil fuels. Fossil fuels are are natural oils and gasses that have been compounded within the Earth’s crust under great pressure and over long periods of time. Most of these fuels either have a significant carbon component, or release carbon dioxide when they are burned.This means that much of human activity and industrialization has had a negative impact on carbon emissions and ocean acidification. When fuels such as natural gas, oil, or coal are burned, the carbon dioxide is released into the atmosphere where it then cycles back and is absorbed by the ocean. While the carbon cycle never increases or decreases its carbon amounts, humans are burning and releasing carbon much more quickly than it can be naturally absorbed. Fossil fuels take thousands of years to form, but humans have been burning them at rapid rates over the last 200 years or so.

Additionally, the main natural carbon sink, or carbon absorption system, is forest plants and other flora. Humans have also had a negative impact in this regard, as deforestation and tree clearing has meant the removal of large areas of vegetation which used to serve as carbon sinks. Plants naturally absorb and retain carbon dioxide, so the decrease in plant life means the carbon must be absorbed in other ways. This is one of the major reasons so much of the excess carbon dioxide in the atmosphere is absorbed by oceans and large bodies of water.
Effects Of Acidification On Marine Life
An increase in pH level - even a slight increase - can be very harmful to marine life and marine ecosystems. Acidic water can be especially harmful to shellfish. Many crustaeans and shelled marine animals rely on carbonate ions to build their shells. Carbonate and calcium are combined to form coral and shells, but when the ocean levels become more acidic, carbonate ions bond with excess hydrogen. Without the needed carbonate, shells either cannot form, or do not form properly, leaving these sea creatures vulnerable to predators.
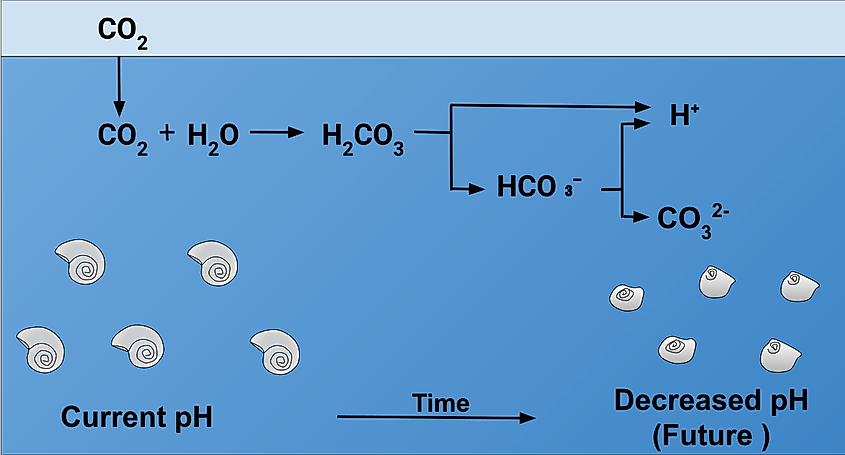
Similarly, acid is an eroder, and overly acidic water will eat away at these very same calcium-carbonate compounds, such as the coral reefs or oyster and mollusc shells. Changes in the lower levels of food chains- such as in the case of small crustaeans or snails - can have massive effects on the rest of the system. While small shellfish may not seem vital, they are food sources for other large marine life - including species of fish which humans eat in large quantity. Not only can acidification break down the system, but it can throw entire biomes off balance, leading to species overpopulation or extinction, and irreparable changes to food chains.
What Can We Do?

Because so much of ocean acidification is linked to carbon emissions, focusing on reducing carbon footprints and air pollution can help to slow this acidification process. Similarly, chemical waste that drains directly into water systems - whether agricultural or industrial should be curbed. In addition, limiting large-scale agriculture and deforestation can also help with the acidification issue. Maintaining natural carbon sinks, such as forests, will ensure there are natural systems in place to filter and retain some of the excess carbon dioxide.