Why Does Ice Float?
When something is less dense than water, it will inevitably float when placed in water. Since ice floats, it must be less dense than liquid water. Interestingly, the fact that water is less dense in its solid state than its liquid state makes it a very unique compound. Most compounds and elements are denser in their solid state than their liquid state, yet water is an exception. Water is at its highest density at a temperature of 4 degrees Celsius. Moreover, ice weighs about 90% of its volume of water, therefore, 90% of the ice will be under water and the rest above. Generally, when a compound is in a solid state, individual molecules are packed together more tightly compared to when that compound is in its liquid state, and so the solid state is comparatively denser than the liquid state. Why is this not the case for water?
Hydrogen Bonds
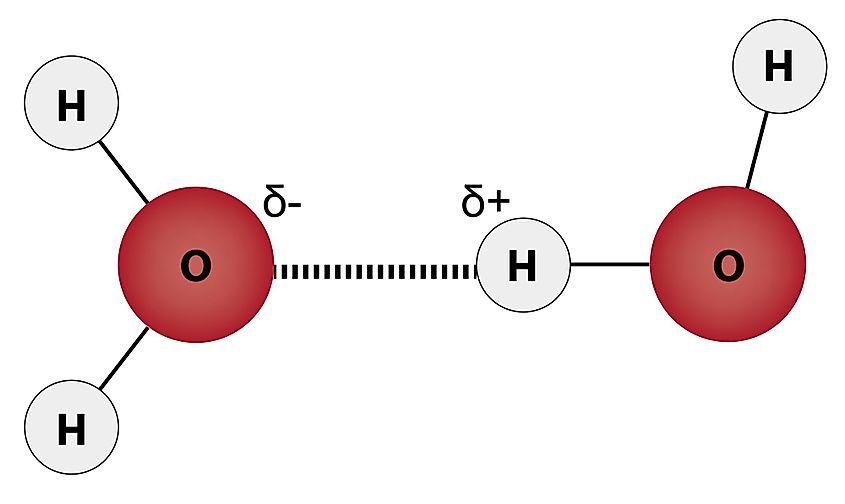
The reason why ice floats on water has due to something called hydrogen bonding. A water molecule is made up of one oxygen atom at the center and one hydrogen atom on each side. The entire water molecule is held by covalent bonds, meaning that electrons are being shared between the oxygen and hydrogen atoms. The oxygen atom pulls on the hydrogen’s electrons more strongly, giving the oxygen a negative charge and leaving the hydrogen with a slight positive charge. Since opposite charges attract, hydrogen and oxygen are drawn to one another, which is what hydrogen bonding is.
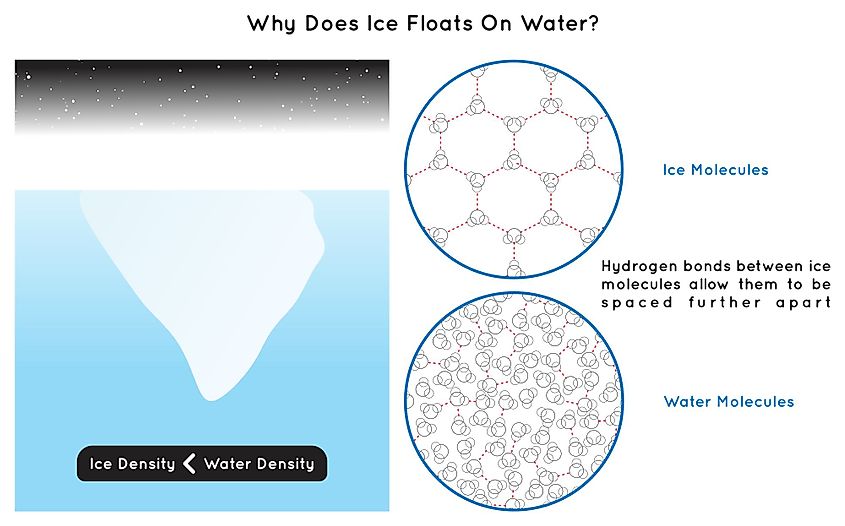
When water begins to freeze, water molecules form an orderly crystal structure. Usually, when water is liquid, individual water molecules can move past each other, with opposite charges attracting one another. However, when water freezes, the orientation of water molecules causes identical charges to repel one another. This means that water molecules are pushed apart when frozen, decreasing the overall density of ice. Since the molecules are pushed apart, the frozen water will actually expand. As the frozen water expands, it creates a force that pushes outwards. This is the reason why containers and other objects can break if water is frozen within them.
Significance Of Ice Floating On Water

Life on Earth would be rather difficult if ice did not float on water. For example, when bodies of water freeze in colder regions, only the top layer freezes, and below the ice, water still exists in liquid form. This is because ice floats on liquid water, and by forming a thick layer of ice, it insulates the liquid water below. If ice were denser than liquid water, it would sink to the bottom, and entire bodies of water would completely freeze from the bottom up. This would make it impossible for marine organisms to survive in colder regions, and it would be difficult for our planet to maintain a stable ecosystem. Thankfully, ice is less dense than water, and life can flourish as such.